Understanding the Concept of Equilibrium, Balance and its Applications
What is Equilibrium?
In other words equilibrium refers to a condition whereby something attains a stable state or is in a balanced condition. It is the condition where all the forces which are at work leading to activities are equal and balanced. Hence the system is balanced and has zero tendency to change in the future.
Types of Equilibrium
The concept of equilibrium extends far beyond a simple physics problem. We encounter various forms of equilibrium:
- Mechanical Equilibrium: Balance of forces and torques (turning forces)
- Thermal Equilibrium: The heat flow is balanced, making both objects have equal temperature.
- Chemical Equilibrium: Steady state between the rate of forward reaction and the rate of reverse reaction in a chemical reaction.
- Economic Equilibrium: Balance of supply and demand in markets (though achieving true equilibrium in economics is a complex endeavor!)
Importance of Equilibrium
Understanding equilibrium is crucial for:
Predicting System Behavior:
Equilibrium is very important in that it helps one to guess the next course of the system.
Designing Stable Structures:
Indeed, engineers apply the principles relating to equilibrium for designing the elements of civil structures like bridges and buildings.
Optimizing Processes:
Chemical engineers work on chemical balances, to achieve the best results of the chemical reactions that occur.
Understanding Natural Phenomena:
We find the basis of equilibrium in every weather forecast, ocean currents, and even in stabilization of stars.
Mechanical Equilibrium, Examples and Applications
Definition and Concepts:
Static Equilibrium: An object that has no acceleration and is at equilibrium with no net force and no net torque on it. Think about a book lying on a table or a painting that has been hanged and does not move.
Dynamic Equilibrium: An object in motion with constant velocity (no change in speed or direction), meaning the net force and torque are also zero. Think of a car cruising at a steady speed on a straight highway.
Conditions for Mechanical Equilibrium:
- Net Force = 0: The vector sum of all the forces acting on an object must be equal to zero.
- Net torque= 0 : The vector sum of all torques of an object is always equal to zero.
Examples in Everyday Life:
Balanced Seesaw:
If two weights of equal size are located at the equal distance from the fulcrum then the condition of the system is known to be balanced.
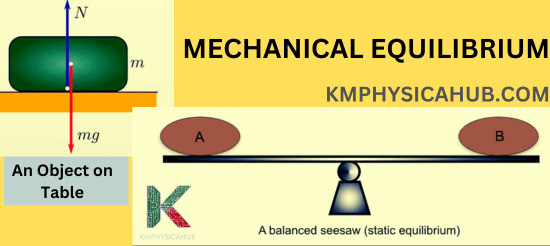
Stationary Object:
A book placed on a table is supposed to be at rest – the forces exerted on the book are opposite yet equal. The downward force known as gravity is always in equilibrium with another force known as the normal force which is exerted through the table.
Pendulum at Rest:
An example of a good and stable equilibrium position of a pendulum is its vertical position. It is free to move horizontally either way; however, it ceases to oscillate after sometime when a slight perturbation is applied to it.
Applications:
Engineering:
Build structures like bridges, buildings etc. that can support loads and stress.
Architecture:
The design of beautiful and functional, weight-bearing structures and the construction and management of such structures.
Biomechanics:
Observing people’s full spectrum of motion and constructing artificial limbs.
Thermal Equilibrium, Examples and Applications
Definition and Concepts:
Heat Transfer: The transfer of heat energy from one point of higher magnitude to another of lower magnitude.
Temperature: The term Temperature refers to “Average kinetic energy of particles” in a system.
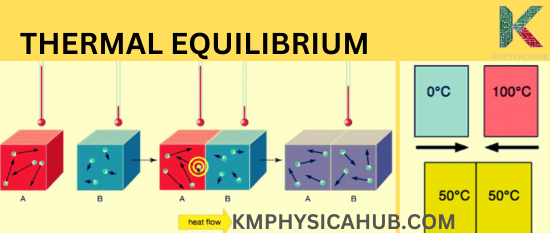
The zeroth law of thermodynamics: If two systems A and B are in thermal equilibrium with a third system C, then both systems A and B are always in thermal equilibrium with each other. This law is used for temperature measurement.
Examples in Everyday Life:
Coffee Cooling Down:
Pouring a cup of coffee, then leaving it on the counter will also cool down to the room temperature, hence coming to the thermal equilibrium with the room.
Melting Ice:
Ice taken from its normal storage then exposed to a warmer condition will undergo melting and vaporization in its effort to attain a temperature balance with the external temperature.
Applications:
Climate Science: Learning about heat converged or dispersed in the earth’s atmosphere and the ocean.
Material Science: Estimating the possible behavior of material and creating materials with required thermal characteristics.
Heat Engines: Creating engines for converting thermal energy to mechanical energy like the inner combustion engines and power plants.
Chemical Equilibrium, Examples and Applications
Definition and Concepts:
Reversible Reactions:
While some reactions are strictly unidirectional, there exist numerous cases where reactions are reversible. This means that product formed in the reaction can also undergo further reaction to form reactants again. For example, consider a case where there is a two-line highway where vehicles can move in both ways.
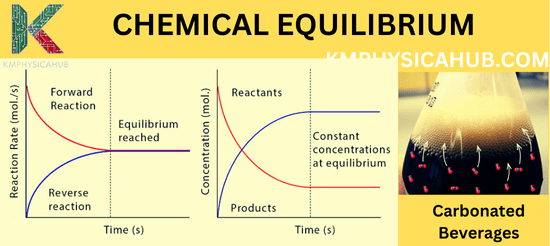
Equilibrium Constant (K):
At equilibrium, the number of real events for the forward and reverse reactions is the same, and there is no further change in the concentrations of the reactants and products (though they are not necessarily equal). The equilibrium constant (K) is a ratio, which describes this situation and contains information about the degree of a reaction’s completion.
Factors Affecting Chemical Equilibrium (Le Chatelier’s Principle):
Any change to stability is not embraced by a system at equilibrium and therefore it will try to counter it. Le Chatelier’s Principle is expressed that if the change in conditions is applied to the system which is at equilibrium, the system will change in the direction which reduces the effect of stress. It is also important to note that factors such as concentration variation, change in temperature, and change in pressure affect the position of the equilibrium.
Examples in Everyday Life:
Dissolving Sugar in Water:
In the forward reaction of water to sugar, the sugar dissolves into the water. But, if you add enough sugar, some of it will not mix with the water and this remains a dynamic equilibrium, some sugar dissolving and some not.
Carbonated Beverages:
The bubbles that make your soda pop are CO2 dissolved in liquid in your bottle, and CO2 gas in the headspace. When a bottle is opened it alters the pressure, and this shifts the balance to the extent that the CO2 dissolved in the liquid starts becoming bubbles.
Applications:
Chemical Industry: This process involves changing chosen parameters such as temperature, pressure, and the use of catalysts to get the highest yields of wanted products such as ammonia for fertilizer or plastics.
Environmental Science: Chemical equilibrium is important in the understanding of such environmental factors like solubility of pollutants in water or density of gasses in the atmosphere.
Biological Processes: All biochemical reactions occurring in our bodies from oxygen binding by hemoglobin to the regulation of enzymes involve the ability to balance things.
Economic Equilibrium, Examples and Applications
Definition and Concepts:
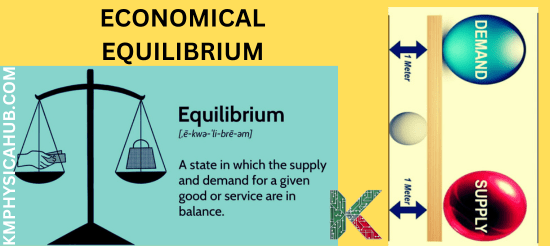
When dealing with the concept of goods and services in the marketplace, equilibrium refers to the situation that shows the ability of buyers to buy and sellers to sell the products at the current market price.
Supply is the quantity of the given products that is available in the market which producers are willing to offer at that certain price level. Every time the price rises, the producer is motivated to produce more of the product in question.
Where, demand is the amount of a good or service that a consumer is willing to take at the prevailing price. Normally when the price is high, the quantity demanded in the market is low and vice versa.
In most of the situations in a free economic world, to maintain equilibrium, the quantity supplied is equal to quantity demanded in a given market.
Examples in Everyday Life:
Suppose there is the launch of a new model of the smartphone and this leads to high demand. The first few quantities may be scarce, probably due to a lack of availability, and thus expensive. When production increases to fulfill this demand, the price will gradually reach an average price level to satisfy the quantity demanded with the quantity supplied.
Applications:
Macroeconomic Analysis: Equilibrium models in economics are commonly used when the basic condition of an economy or the entire economy is under consideration, number of factors being considered include inflation, unemployment and economic growth.
Financial Markets: Market equilibrium principles assist in explaining how and at what cost financial demands of stock, interest, and exchange rates are met.
Policy Decisions: Government policies concerning taxes, subsidies and regulations can alter supply and demand; this changes the position of equilibrium in the market. By knowing these dynamics it becomes easier for the policy maker to make the right decisions.
Equilibrium in Nature: A Delicate Balancing Act
Natural Systems and Equilibrium:
Nature is unique in its ability to balance all its elements as it is pulling together harmony in all it systems. From and ecosystem’s food chain to geophysical phenomena, balance has been depicted as the central force.
Ecosystems:
If a balance is kept in an ecosystem, then you find that different species of animals will be living in a balanced number. This balance is kept by predator-prey relationships, availability of resources and other factors affecting the environment.
Weather Patterns:
This is very complex because our planet has a changing atmosphere and ocean system but the weather system provides a balance over certain periods. Additionally, amount of solar energy received at earth’s surface and also, emittance of heat from the surface, atmospheric and oceanic circulation participate in the balance.
Geological Formations:
Even what appears to be geologically fixed in nature can be subject to the forces of equilibrium. Processes such as tectonic uplift and erosion for instance determine the formation of mountains and valleys over millions of years.
Examples:
Predator-Prey Balance:
Think about wolves and deer as interacting species within the geographic space of a forest. The increase in the number of deer increases the food factor for wolves thus increasing the number of wolves. But, this increases predation until the deer numbers are reduced, which in turn reduces the number of wolves to set up a balance again.
Carbon Cycle:
Equilibrium at the global level is seen with the case of the carbon cycle in the Earth. Carbon moves within and between the atmosphere, ocean, living things, and rocks. Man made activities have interfered this balance while the natural ones like photosynthesis and respiration in organisms help maintain CO2 levels in the atmosphere.
Disruptions and Shifts:
The condition of natural balance can be disturbed by external influences, the effects of which may be catastrophic:
Climate Change:
Climate change caused by human intervention is a perfect instance of disturbance within the Earth’s balance, thus resulting to increased temperatures, changes in weather and environments.
Habitat Loss:
Habitat destruction and the separation of organisms from each other are the main reasons for the imbalance of the ecosystem, which leads to species extinction and the inconsistency of the ecosystem.
Pollution:
Pollution of air, water, and soil by human activities is comparable to a stone thrown into a pond, the effects would cause the natural system’s disruption, and then the whole ecological system to be affected which consequently threatens the health of living beings.
Conclusion:
Beginning with the concrete forces that construct our physical environment and extending through the complexities of natural systems and the behavior of economic markets, balance is the unifying principle. Equilibrium is an essential topic in comprehending the dynamics of the world and its nature since it can assist in predicting phenomena in various disciplines, ranging from economics to environmental issues.
When exploring the various mysteries of the universe, the microcosm, and the macrocosm, the case of equilibrium shall continue to be a very crucial element in human discovery in the years to come.
People Also Ask
Q1. What is equilibrium?
A: Equilibrium describes a condition where there are two equal and opposite forces at work ensuring that there is no change in state.
Q2. What are the main types of equilibrium?
A:
- Mechanical Equilibrium
- Thermal Equilibrium
- Chemical Equilibrium
- Economic Equilibrium
Q3. What are the conditions for mechanical equilibrium?
A:
- The Sum of all the forces must be zero to make the forces balanced.
Net Force = 0
- The Sum of all torques must be zero to make the torques balanced.
Net torque= 0 : The vector sum of all torques of an object is always equal to zero.
Q4. Give three examples of equilibrium in everyday life?
A:
A balanced seesaw
A stationary object on a table
Simple pendulum at rest